Abstract
Background
Carfilzomib (K) and bortezomib (V) and have been studied in head-to-head comparison using a K dose of 56mg/m2 in doublet with dexamethasone at relapse (ENDEAVOR), and also at 36mg/m2 in triplet with melphalan and prednisolone in newly diagnosed non-transplant eligible patients. Differing results may relate to dosing and scheduling, as well as to different study populations. Triplet regimens at relapse are commonly used, and combinations with cyclophosphamide and dexamethasone (Cd) are relatively affordable.
Aims
The MUK five phase 2 study compared the activity and safety of K (36mg/m2 ) and V in triplet combination with Cd for patients at first relapse, or refractory to no more than 1 prior line of therapy, and investigated the benefit of K maintenance following fixed duration KCd.
Methods
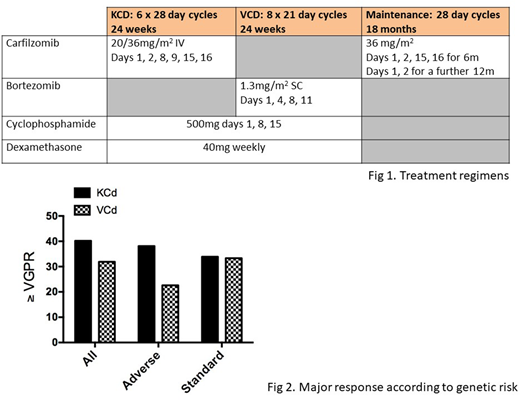
Eligible patients were randomized (R1) 2:1 to receive 6 cycles of KCd or 8 cycles of VCd (Fig 1); patients with ≥SD at the end of KCd were randomized (R2) 1:1 to receive maintenance K (Fig 1) or no further treatment. Patients in the VCd arm did not receive maintenance. Co-primary endpoints were ≥VGPR rates at 24 weeks post R1 (powered for non-inferiority, 90% confidence interval (CI), odds ratio (OR) >0.8), and PFS from R2 (superiority, 80% power, HR 0.67). Disease response was assessed by IMWG criteria, and minimal residual disease (MRD) by multiparameter flow cytometry (10-4). Adverse genetic risk was any one of t(4;14), t(14;16), t(14;20), del(17p) or 1qgain.
Results
300 patients were randomized, 201 KCd and 99 VCd. Patient and disease features were balanced between arms (reported ASH 2017). The safety population was 292 patients (KCd, 196; VCd 96). While 81.6% of patients in the KCd arm received all 6 treatment cycles, only 53.5% in the VCd arm received all 8 cycles. Safety data (reported ASH 2017) showed increased treatment emergent neuropathy with VCd (≥G3 or G2 with pain = 19.8% vs 1.5% with KCd), and more cardiac and hypertension ARs with KCd (G3 3.6% vs 0%, 4.1% vs 2.1%). Overall response at 24 weeks KCd vs VCd: ≥PR 84.0% vs 68.1%, OR 2.72, p=0.0014. Maximum response within 12m (with matching adjustment to remove bias of K maintenance) KCd vs VCd: ≥VGPR 53.1% vs 45.4%; ≥PR 91.3% vs 78.9%, diff=12.4%, 90% CI (2.0, 22.6). Maximum response overall KCd vs. VCd: ≥VGPR 53.6% vs 45.4%.
In an updated analysis of genetic data (228 patients), 64/152 (42.1%) patients in KCd arm had adverse risk, vs 34/76 (42.1%) in VCd arm. 79.7% and 65.6% patients with adverse risk completed all cycles of KCd and VCd, respectively, compared to 92.9% and 40.0% patients with standard risk. In the KCd arm, 38.1% of adverse risk patients achieved ≥VGPR at 24 weeks vs 33.9% with standard risk. In the VCd arm, ≥VGPR was 22.6% in adverse risk vs 33.3% in standard risk patients (fig 2).
PFS comparisons were based on a 10% 1-sided significance level. For the R1 comparison, 100 (51.0%) and 71 (74.0%) patients have progressed/died in the KCd and VCd arms respectively from R1. Median PFS for KCd (patients receiving maintenance censored) was 11.9m (80%CI 11.6, 12.7) vs 10.2m (9.3, 11.3) for VCd, p=0.50 (not powered to detect difference). A Cox multivariate model for PFS including treatment arm, b2m, previous V, previous ASCT and timing of relapse showed independent effect of b2m level and previous ASCT only. OS data are immature, with only 25% events in each arm.
Of 141 patients eligible for R2, 69 were allocated to maintenance K. Arms were well balanced for response (≥VGPR: K 58.0%, obs 54.2%) ECOG, ISS, genetic risk and MRD status (MRD-neg: K 11.6%, obs 13.9%) at end of initial treatment. Safety and tolerability of maintenance have previously been reported (EHA 2018). Median follow-up for patients from R2 was 10.5m (0.9-31.3): 44.3% of patients completed 6 cycles K maintenance. 82.1% of patients had a dose modification. K maintenance was associated with a longer PFS from R2, median 11.9m vs 5.6m obs (HR 0.59, p=0.009). The combined PFS of patients receiving KCd induction followed by K maintenance was 18.1 months (80% CI 14.4-18.9).
Conclusion
In patients with adverse genetic risk, KCd achieves higher ≥VGPR rate compared to VCd. This contributes to the higher ORR seen with KCd. Maintenance K was associated with longer PFS. Use of KCd triplet for fixed duration followed by maintenance K resulted in combined PFS of 18.1m, which is comparable to the median PFS for patients in ENDEAVOR who had 1PL (22m). The effect of genetic risk on survival outcomes will be updated at the meeting.
Yong:Celgene: Honoraria; Takeda: Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau. Williams:Amgen: Honoraria, Speakers Bureau; Celgene: Honoraria, Other: travel support, Speakers Bureau; Novartis: Honoraria; Janssen: Honoraria, Other: travel support, Speakers Bureau; Takeda: Honoraria, Other: travel support, Speakers Bureau. Cavenagh:Celgene: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Kaiser:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Other: travel support; Chugai: Consultancy; Bristol-Myers Squibb: Consultancy, Other: travel support; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Rabin:Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Takeda: Consultancy, Other: Travel support , Speakers Bureau; Janssen: Consultancy, Other: Travel support, Speakers Bureau. Ramasamy:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Garg:Novartis: Other: travel support, Research Funding; Amgen: Honoraria, Other: Travel Support; Janssen: Honoraria; Takeda: Other: Travel Grant. Auner:Takeda: Consultancy; Amgen: Honoraria, Research Funding; Novartis: Consultancy; PharMar: Consultancy. Hawkins:Takeda: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Bygrave:Celgene: Honoraria, Other: Meeting sponsorship; Janssen: Honoraria, Other: Meeting sponsorship; Takeda: Honoraria, Other: Meeting sponsorship; Novartis: Honoraria. Morgan:Janssen: Research Funding; Takeda: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria. Davies:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; ASH: Honoraria; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Janssen: Consultancy, Honoraria; MMRF: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TRM Oncology: Honoraria. Owen:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Other: Travel Support; Janssen: Consultancy, Other: Travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal